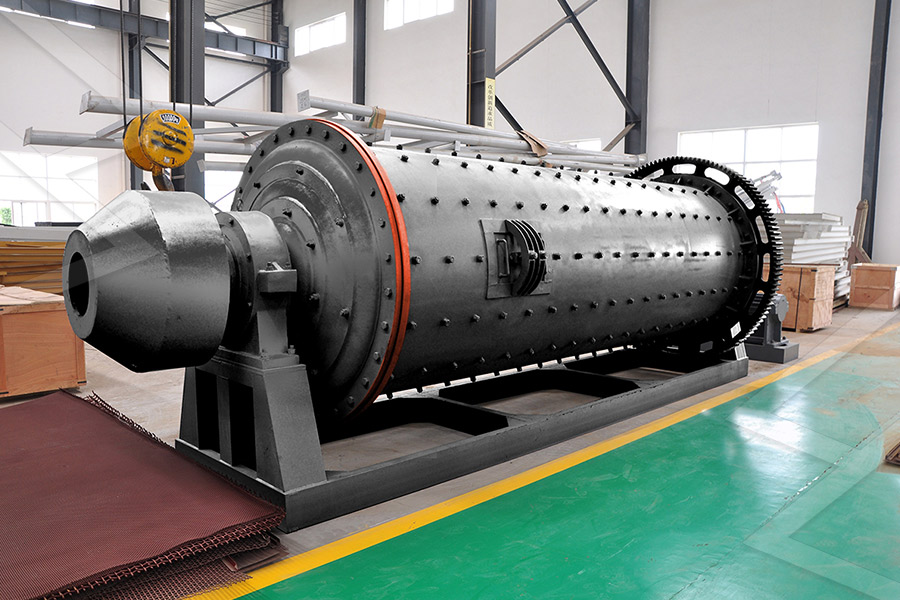
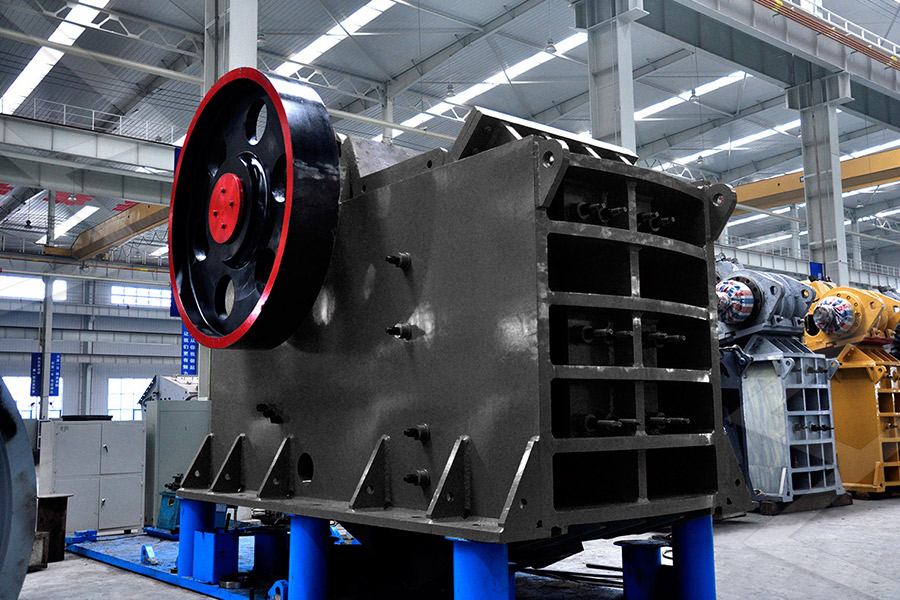
产品世界
产品系列完备,打通粗碎、中碎、细碎和超细碎作业
VSI家族与VU骨料优化系统共担精品机制砂制备重任
zn
 2022-05-31T13:05:24+00:00
2022-05-31T13:05:24+00:00

Zinc Element information, properties and uses Periodic
Represented in the periodic table as Zn, zinc is a transition metal, grouped with cadmium and mercury With the middling atomic number 30, it has five stable isotopes of atomic May 2, 2023 zinc (Zn), chemical element, a lowmelting metal of Group 12 (IIb, or zinc group) of the periodic table, that is essential to life and is one Zinc Properties, Uses, Facts BritannicaZinc is a chemical element with the symbol Zn and atomic number 30 Zinc is a slightly brittle metal at room temperature and has a shinygreyish Zinc Wikipedia

Zinc group element chemistry Britannica
zinc group element, any of the four chemical elements that constitute Group 12 (IIb) of the periodic table—namely, zinc (Zn), cadmium (Cd), mercury (Hg), and copernicium (Cn) They have properties in common, but they Get the latest 10 Year US Treasury Notes price (ZN) as well as the latest futures prices and other commodity market news at Nasdaq10 Year US Treasury Notes (ZN) Nasdaq54 rows Naturally occurring zinc ( 30 Zn) is composed of the 5 stable isotopes 64 Zn, 66 Zn, 67 Zn, 68 Zn, and 70 Zn with 64 Zn being the most abundant (486% natural Isotopes of zinc Wikipedia

Zion Oil Gas Stock Forecast, Price News
May 5, 2023 Zion Oil Gas' mailing address is 12655 NORTH CENTRAL EXPRESSWAY SUITE 1000, DALLAS TX, 75243 The official website for the company is Jul 13, 2020 This chemistry video tutorial explains how to predict the products of the single replacement reaction between Zinc and Hydrochloric Acid It explains how toZn + HCl Reaction Zinc + Hydrochloric Acid YouTubeMay 9, 2021 Figure \(\PageIndex{3}\): Determining a Standard Electrode Potential Using a Standard Hydrogen Electrode The voltmeter shows that the standard cell potential of a galvanic cell consisting of a SHE and a 62: Standard Electrode Potentials Chemistry LibreTexts

ZN Zion Oil Gas Inc Stocktwits
ZN Stock Price Zion Oil Gas, Inc engages in the exploration of oil and natural gas properties The company was founded by John M Brown on April 6, 2000 and is Represented in the periodic table as Zn, zinc is a transition metal, grouped with cadmium and mercury With the middling atomic number 30, it has five stable isotopes of atomic weight from the dominant zinc 64 to zinc 70, plus an extra 25 radioisotopesZinc Element information, properties and uses Periodic Tablezinc group element, any of the four chemical elements that constitute Group 12 (IIb) of the periodic table—namely, zinc (Zn), cadmium (Cd), mercury (Hg), and copernicium (Cn) They have properties in common, Zinc group element chemistry Britannica

Zinc Zn (Element) PubChem
Chemical element, Zinc, information from authoritative sources Look up properties, history, uses, and moreZinc (Zn) Zinc is the 30th element on the periodic table Zinc is a bluishwhite metal that is used to make other metals The symbol is 'Zn' Know the Uses of Zinc, Properties of Zinc and more at BYJU'SUses of Zinc (Zn) Atomic Data, Chemical PropertiesGet the latest 10 Year US Treasury Notes price (ZN) as well as the latest futures prices and other commodity market news at Nasdaq10 Year US Treasury Notes (ZN) Nasdaq

Isotopes of zinc Wikipedia
Naturally occurring zinc ( 30 Zn) is composed of the 5 stable isotopes 64 Zn, 66 Zn, 67 Zn, 68 Zn, and 70 Zn with 64 Zn being the most abundant (486% natural abundance ) Twentyfive radioisotopes have been characterised with the most abundant and stable being 65 Zn with a halflife of 24426 days, and 72 Zn with a halflife of 465 hoursAtomic Number – Protons, Electrons and Neutrons in Zinc Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleusTotal number of protons in the nucleus is called the atomic Zinc Periodic Table and Atomic PropertiesZinc Zn CID 23994 structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards Zinc Zn PubChem

Zinc Electron Configuration and Oxidation States Zn
Nov 13, 2020 Zinc is a chemical element with atomic number 30 which means there are 30 protons and 30 electrons in the atomic structureThe chemical symbol for Zinc is Zn Electron Configuration and Oxidation States of Zinc Electron configuration of Zinc is [Ar] 3d10 4s2 Possible oxidation states are +2 Electron Configuration The periodic table is May 9, 2021 Figure \(\PageIndex{3}\): Determining a Standard Electrode Potential Using a Standard Hydrogen Electrode The voltmeter shows that the standard cell potential of a galvanic cell consisting of a SHE and a 62: Standard Electrode Potentials Chemistry When zinc metal is submerged into a quantity of aqueous HCl, the following reaction occurs (Figure 54 "Zinc Metal plus Hydrochloric Acid"):Zn(s) + 2HCl(aq) → H 2 (g) + ZnCl 2 (aq) This is one example of what is OxidationReduction (Redox) Reactions GitHub Pages

Electron Configuration for Zinc and Zinc ion(Zn2+)
The zinc atom donates two electrons in the 4s orbital to form a zinc ion (Zn 2+ ) Zn – 2e – → Zn 2+ Here, the electron configuration of zinc ion (Zn 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 Zinc atom exhibit +2 oxidation state The oxidation state of the element changes depending on the bond formationZinc hydroxide Zn ( OH) 2 is an inorganic chemical compound It also occurs naturally as 3 rare minerals: wülfingite (orthorhombic), ashoverite and sweetite (both tetragonal) Like the hydroxides of other metals, such as lead, aluminium, beryllium, tin and chromium, Zinc hydroxide (and Zinc oxide ), is amphotericZinc hydroxide WikipediaWord Equation One mole of solid Zinc [Zn] and two moles of aqueous Hydrogen Chloride [HCl] react to form one mole of aqueous Zinc Chloride [ZnCl2] and one mole of Dihydrogen [H2] gas For some metals Zinc (Zn) into the bottom tube, add 12 Ml tube acid solution → Dark gray solids Zinc (Zn) melting and cavitation due to hydrogen gas (H2) formedZn + HCl = ZnCl2 + H2 Chemical Equation Balancer

Zinc group element chemistry Britannica
zinc group element, any of the four chemical elements that constitute Group 12 (IIb) of the periodic table—namely, zinc (Zn), cadmium (Cd), mercury (Hg), and copernicium (Cn) They have properties in common, Chemical element, Zinc, information from authoritative sources Look up properties, history, uses, and moreZinc Zn (Element) PubChemAtomic Number – Protons, Electrons and Neutrons in Zinc Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleusTotal number of protons in the nucleus is called the atomic Zinc Periodic Table and Atomic Properties

OxidationReduction (Redox) Reactions GitHub Pages
When zinc metal is submerged into a quantity of aqueous HCl, the following reaction occurs (Figure 54 "Zinc Metal plus Hydrochloric Acid"):Zn(s) + 2HCl(aq) → H 2 (g) + ZnCl 2 (aq) This is one example of what is The zinc atom donates two electrons in the 4s orbital to form a zinc ion (Zn 2+ ) Zn – 2e – → Zn 2+ Here, the electron configuration of zinc ion (Zn 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 Zinc atom exhibit +2 oxidation state Electron Configuration for Zinc and Zinc ion(Zn2+)Zinc hydroxide Zn ( OH) 2 is an inorganic chemical compound It also occurs naturally as 3 rare minerals: wülfingite (orthorhombic), ashoverite and sweetite (both tetragonal) Like the hydroxides of other metals, such as lead, aluminium, beryllium, tin and chromium, Zinc hydroxide (and Zinc oxide ), is amphotericZinc hydroxide Wikipedia

Zinc bromide Wikipedia
Zinc bromide (Zn Br 2) is an inorganic compound with the chemical formula Zn Br 2It is a colourless salt that shares many properties with zinc chloride (ZnCl 2), namely a high solubility in water forming acidic solutions, and Word Equation One mole of solid Zinc [Zn] and two moles of aqueous Hydrogen Chloride [HCl] react to form one mole of aqueous Zinc Chloride [ZnCl2] and one mole of Dihydrogen [H2] gas For some metals Zinc (Zn) into the bottom tube, add 12 Ml tube acid solution → Dark gray solids Zinc (Zn) melting and cavitation due to hydrogen gas (H2) formedZn + HCl = ZnCl2 + H2 Chemical Equation BalancerThe FOMC, at its November 2021 meeting, announced that it would begin tapering QE by $15 billion a month starting in midNovember A surge in inflation expectations also undercut Tnote prices after the 10year breakeven inflation rate soared to a 161/2 year high of 278% in November Tnote prices tumbled to a 2year low in November10Year TNote Jun '23 Futures Contract Specifications Barchart

ZN Zion Oil Gas Stock Price Barchart
ZN Stock Quotes API Business Summary ZION OIL GAS, INC was founded for the purpose of exploring for oil and gas in Israel and reaching production levels that would help Israel become an energy independent countryAug 20, 2004 Structural evidence for the absence of hydride bridges is provided by the observation that the zinczinc bond length in Cp* 2 Zn 2 is 231 Å, much shorter than the value of 245 Å in a known hydridebridged dimer ( 8 ) For further comparison, the cadmiumcadmium bond length in Cd 2 (AlCl 4) 2 is 258 Å ( 5 ), and mercurymercury ZincZinc Bonds: A New Frontier ScienceZinc (Zn) Zinc is the 30th element on the periodic table Zinc is a bluishwhite metal that is used to make other metals The symbol is 'Zn' Know the Uses of Zinc, Properties of Zinc and more at BYJU'SUses of Zinc (Zn) Atomic Data, Chemical Properties Facts about Zinc

Electronegativity of zinc vs copper in galvanic cell
Nov 21, 2012 It takes more energy to remove two electrons from copper than to remove two electrons from zinc Thus, to get a negative energy change (spontaneous), when need to add the zinc reaction as written and the copper reaction in reverse: Z n Z n 2 + + 2 e X − Δ H = 2692 × 10 3 kJ/mol C u 2 + + 2 e X − C u ( s) Δ H = − 2703 × 10 3 kJ/mol64 Zn is the most common isotope, with 4863% of naturally occurring Zinc This isotope has a halflife of 43x10 18 years This is so long, that its radioactivity can be ignored Similarly, 70 Zn (06%), with a half life of 13x10 16 years is usually considered to not be radioactiveZinc Simple English Wikipedia, the free encyclopedia
集团新闻
铜矿可以作制砂原材料吗?
2022-10-28竖井开拓优缺点
2022-03-26石墨制品厂
2021-12-10云南400mm乘600mm型破碎机价云南400mm乘600mm型破碎机价云南400mm乘600mm型破碎机价
2021-01-24福建大田石料破碎机
2020-01-04时产9001500吨石英山石制砂机
2021-11-13做采石场储量报告需要多少钱
2020-05-27粉碎机山东省
2020-10-19小型家庭用磨粉机价格
2021-03-10重晶石选矿设备 广西厂家
2021-10-15玄武岩细石料
2019-05-26立磨入口温度低是什么原因啊
2020-05-16膨胀石墨生产线厂家
2022-06-19立磨加载站电磁阀工作原理视频
2021-06-20蛭石珍珠岩的处理方法
2022-02-05江西河运怎么去广东南沙码头吗
2021-02-20煤立磨电气控制
2019-11-08煤矿设备报价
2021-01-08节能墙体砖用破碎机规格节能墙体砖用破碎机规格节能墙体砖用破碎机规格
2019-01-21湖南省衡阳采石场湖南省衡阳采石场湖南省衡阳采石场
2021-10-28膨润土的粉碎
2023-10-10哪厂生产pcl900b制砂机
2019-10-21立磨规格基本参数及工作原理
2021-08-25国产砂磨机
2021-09-14每小时产1500T悬辊粉砂机
2020-08-19石膏粉生产
2021-04-30圆锥破碎机电流与排矿口
2022-06-02设备垫铁
2019-10-18锆英砂球磨机
2022-01-26奥林丹51含量复合肥价格
2020-05-03